
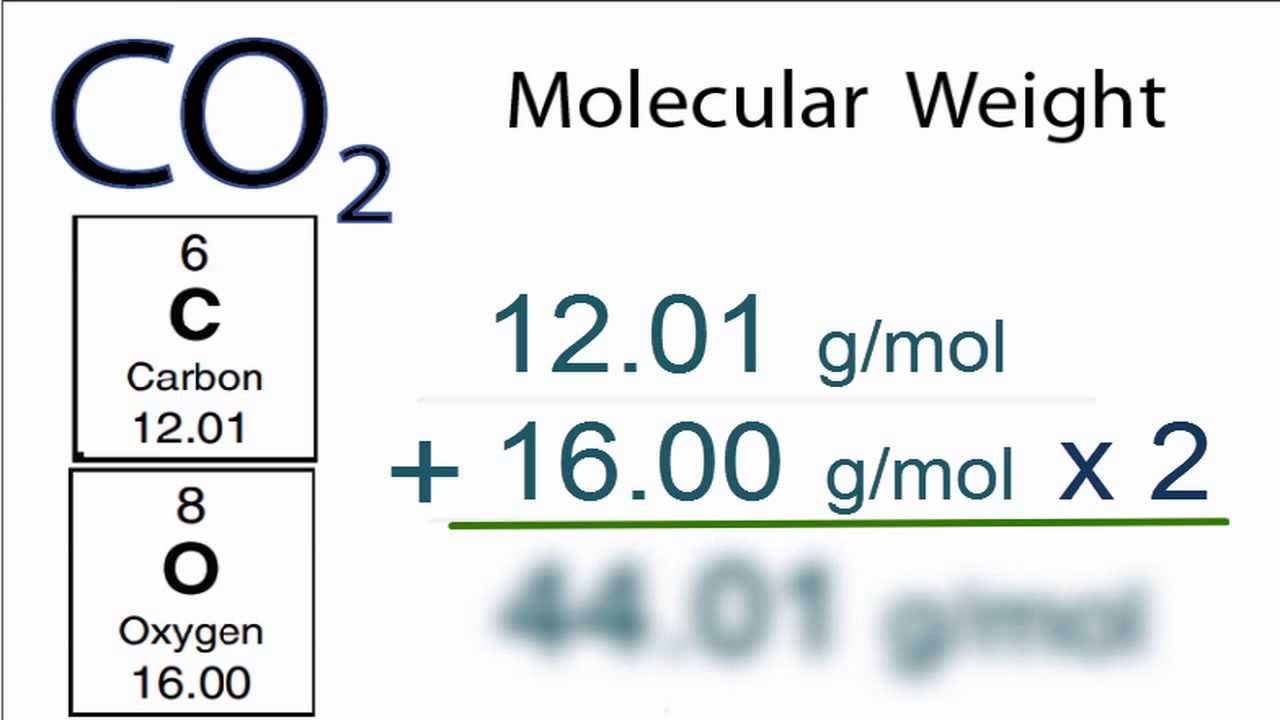

Molecular Orbital (HOMO & LUMO) Visualization.Normal Mode Frequency Analysis with Animation.Activity Score for Nuclear Receptor Ligands.Activity Score for Ion Channel Modulators.Moriguchi Octanol-Water Partition Coefficient (logP).Ghose-Crippen Octanol-Water Partition Coefficient (logP).LogP (Octanol-Water Partition Coefficient).Heat of Vaporization at Normal Boiling Point.Upper Flammability Limit Volume Percent.Lower Flammability Limit Volume Percent.Standard State Gibbs Energy of Formation.The atomic mass takes into account the isotopic distribution of the element in a given sample.įor physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from “Mol-Instincts”, a chemical database based on quantum chemical computations: Molecular masses are calculated from the standard atomic weights of each nuclide, while molar masses are calculated from the atomic mass of each element. Molecular weight is actually an older term of “relative molar mass” or “molecular mass”, which is a dimensionless quantity equal to the molar mass divided by the molar mass constant defined by 1 g/mol. The exact term of the above molecular weight is “molar mass”, which is based on the atomic mass of each element. The molecular weight of Oxygen is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be: The Oxygen molecule consists of 2 Oxygen atom(s) - a total of 2 atom(s).


 0 kommentar(er)
0 kommentar(er)
